It was used to quantitatively detect troponin I in human serum in vitro.
It is used for the auxiliary diagnosis of acute myocardial infarction (AMI).
| 【Product Characteristics】 |
Immunoturbidimetric assay has strong specificity.
Supporting calibration products and quality control products, high detection accuracy.
The reagent is stable for at least 14 days.
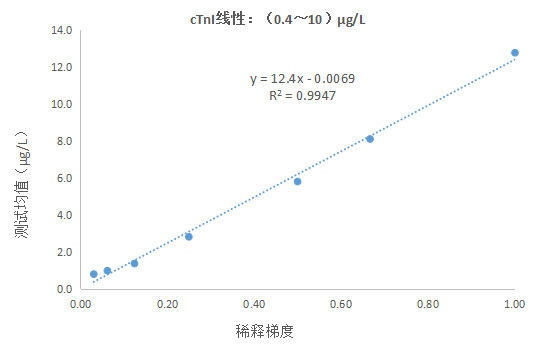
1) linear
2) Repeatability
| n | x(mg/L) | SD | CV(%) |
| Serum 1 | 20 | 2.36 | 0.15 | 6.36 |
| Serum 1 | 20 | 6.01 | 0.28 | 4.66 |
3) Stability
Original package kit: (2-8) C, valid for 12 months
The calibration curve of the reagent can be stabilized for at least 14 days.
4) Reference interval
According to the literature and the company's experimental verification, the reference interval of cTnI is < 1.69 ug/L.
Each hospital should establish its own reference range according to the actual situation in the region.