In vitro diagnostic reagents are uniformly coded nationwide, and each IVD product has a unique “ID card”! The in-vitro diagnostic industry is traceable from production, processing, distribution, distribution, and use. The procurement of reagents and consumables will be unimpeded!
Yesterday (August 27), the State Food and Drug Administration issued the 'Rules for the Unique Identification System of Medical Devices' (hereinafter referred to as the 'Rules'), requiring medical devices that are sold and used in China, and their unique identification systems should comply with these rules. It will be implemented from October 1, 2019.
The 'Rules' said: To implement the 'Notice of the General Office of the State Council on Printing and Reforming High-value Medical Consumables Reform Program' (Guo Ban Fa [2019] No. 37), standardize the construction of a unique identification system for medical devices, and strengthen the life cycle of medical devices. Management, in accordance with the 'Regulations on the Supervision and Administration of Medical Devices', these rules are formulated.
It is worth noting that on August 9th, the State Food and Drug Administration issued the Notice on the Training of the Pilot Work of the Unique Identification System for Medical Devices (hereinafter referred to as the “Notice”), stating that the State Food and Drug Administration took the lead in organizing the medical treatment. Pilot training for unique identification systems for devices.

The first batch of 116 companies participating in the unique identification system pilot medical equipment, Roche, Abbott, Beckman, Siemens, BD, Dean Diagnostics, Antu Bio and other IVD domestic and foreign giants are in the list!
What is the unique identification system for medical devices?
The medical device unique identification system is the identification of the medical device throughout its life cycle. It is the only “identity card” in the product supply chain. It is uniquely identified by the medical device, uniquely identified by the data carrier and uniquely identified by the database. Part of the composition.
The unique identifier of a medical device refers to a code consisting of numbers, letters or symbols attached to a medical device product or package for unique identification of the medical device.
Medical device unique identification data carrier refers to the data medium that stores or transmits the unique identifier of the medical device.
The medical device unique identification database refers to a database that stores product identification and related information uniquely identified by the medical device.
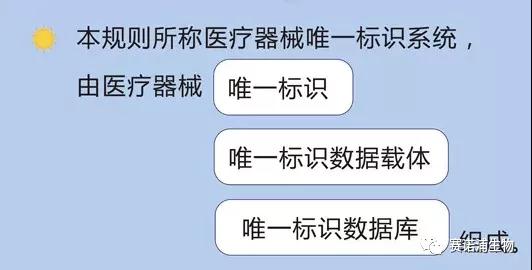
Among them, the unique identifier of the medical device includes the product identification and the production identification.
The product identification is a unique code identifying the registrant/filer, medical device model specifications and packaging; the production identification consists of the code of the medical device production process related information, which may include the medical device serial number, production batch number, according to regulatory and practical application requirements. Production date, expiration date, etc.
How does the unique coding of medical devices work in the in vitro diagnostic industry?
Previously, on June 27th, the State Medical Insurance Bureau issued the 'Notice on Guidance for Medical Standardization Work', which stipulated the code structure of medical insurance medical consumables.
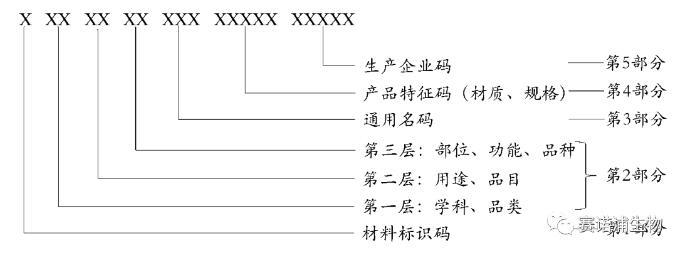
Medical insurance medical consumable code structure
The medical consumable code is divided into 5 parts and 20 parts in total, which are represented by uppercase English letters and Arabic numerals in a specific order. The first part is the consumable identification code, the second part is the classification code, the third part is the common name code, the fourth part is the product feature code, and the fifth part is the production enterprise code.
Part 1: The consumable identification code is indicated by a 1 capital letter 'C'.
Part 2: Classification code, according to the medical consumables discipline, use, location, function division, represented by 6 Arabic numerals.
Part 3: Common name code, create a national unified medical insurance medical supplies common name code, represented by 3 Arabic numerals.
Part 4: Product feature code, code given by characteristics such as consumable material, specifications, etc., expressed in 5 digits.
Part 5: The production enterprise code, which is a unique code given to the consumable manufacturing enterprise based on the medical device registration certificate or the filing voucher, is represented by 5 Arabic numerals.
According to the document, the National Medical Insurance Bureau requires that the medical insurance medical supplies code must have 'uniqueness', forming a 'universal language' for the national medical insurance system to jointly build and share!
It is worth noting that the notice of the National Health Insurance Bureau on June 27 did not explicitly include in vitro diagnostic products. And just over a month, the in vitro diagnostic industry has to try out the unique identification code! This also reflects that the standardization management of the in vitro diagnostic industry by the national government departments is deepening and increasing!
What do IVD companies do?
The 'Rules' show that the standard for the unique identification of medical devices should comply with the relevant standards set by the State Drug Administration and the issuing agencies that meet the requirements of these rules. The registrant/filer shall create and maintain a unique identifier for the medical device in accordance with the standards for the unique identification of the medical device.
The registrant/recorder shall select the data carrier standard appropriate to the unique identification of the medical device it creates, and shall assign a unique identification data carrier to the minimum sales unit of the medical device listed in its name and the higher level packaging or medical device product, and Ensure that the data carrier is uniquely identified, secure, and readable during the operation of the medical device.

The registrant/filer shall upload, maintain and update the relevant data in the unique identification database in accordance with relevant standards or specifications, and be responsible for the authenticity, accuracy and completeness of the data.
The registrant/filer shall submit the product identification in the registration/recording management system when applying for medical device registration, registration change or filing.
The registrant/recorder should upload the product identification and related data to the unique identifier database of the medical device before the product is put on the market.
What are the subsequent effects of the unique identification code for medical devices?
1. Promote the full-scale landing of IVD reagents with “quantity purchase”!
Before the national unified coding, the standard measurement of in vitro diagnostic reagents “purchasing quantity” is a complicated process, because the reagent raw materials, product core components and cost control used by each manufacturer are different.
In the trial of the national code of in vitro diagnostic products, it will be classified according to the five-level classification code, which will be graded, subject, category, use, function, material, specification, manufacturer and minimum packaging specification, and give unique 'IVD reagent consumables'. 'code.
After achieving the national unified coding of in vitro diagnostic medical consumables, it is equivalent to an in vitro diagnostic product with the only “ID card” in the country, which can open the coding, data interface, electronic data exchange, etc. from the inside of the production enterprise to the circulation and use of all links. The interface of relevant information enables the exchange of information throughout the life cycle of medical devices nationwide.
By realizing the unique identification of medical devices, the speed of reagent supply 'purchasing' will be accelerated in the country!
2. Incorporate IVD third-party logistics into the strictest supervision!
The 'Notice on the Training of the Pilot Work of the Unique Identification System for Medical Devices' not only has domestic and international IVD giants, but also many well-known IVD third-party logistics companies such as Sinopharm and Jiuzhoutong!
As early as February 2017, the State Council issued the '13th Five-Year' National Food Safety Plan and the '13th Five-Year Plan for National Drug Safety Planning' clearly stated that the medical device coding rules were formulated and the medical device coding system was constructed. Breaking the situation in which information, such as production, management, use, and supervision, is closed and separate.
In all provinces of China, Shanghai has to use the unique identification code of implantable medical device products in 2006, and upload the hospital use data to the city's unified traceability management system.
In 2011, the third-party logistics storage operation of medical equipment was also included, and the circulation field also became the key regulatory object. Beijing, Hunan, Hebei, and Liaoning have all established electronic monitoring platforms for equipment logistics. Chongqing has established a “two-vote” electronic traceability supervision system to strengthen the supervision of medical equipment circulation traceability.
The trial of the unique device identification for this in vitro diagnostic product will open up the last layer of restrictions and achieve unified supervision of in vitro diagnostic products nationwide and even globally!