On August 1st, the State Food and Drug Administration's Medical Review Center issued the Catalogue of Medical Devices Exempted from Clinical Trials (Revised Draft for 2019) (hereinafter referred to as “Exemption Catalogue (Opinion Draft)” in 2019). 996 medical devices and 420 in vitro diagnostic reagents are exempt from clinical trials.
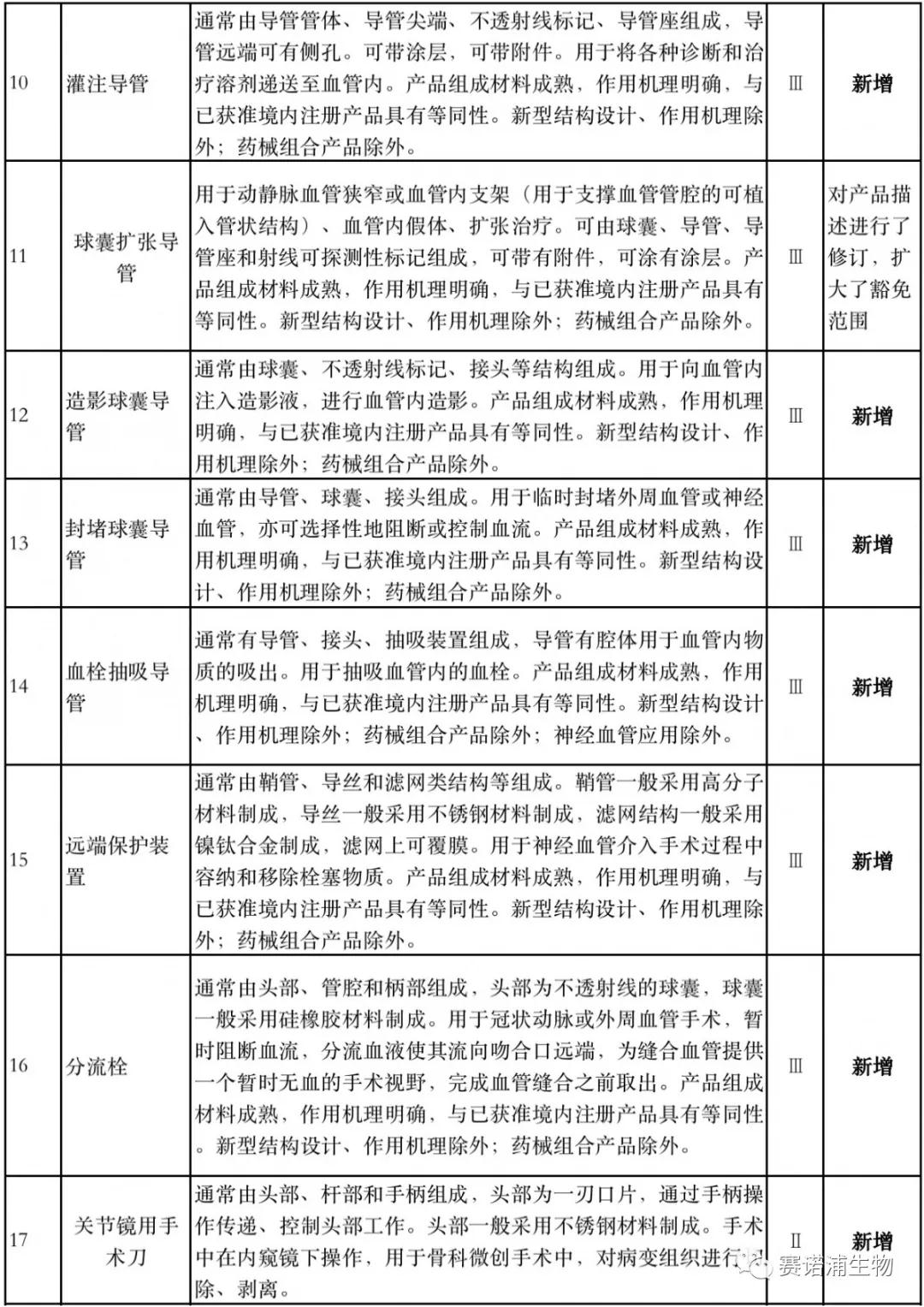
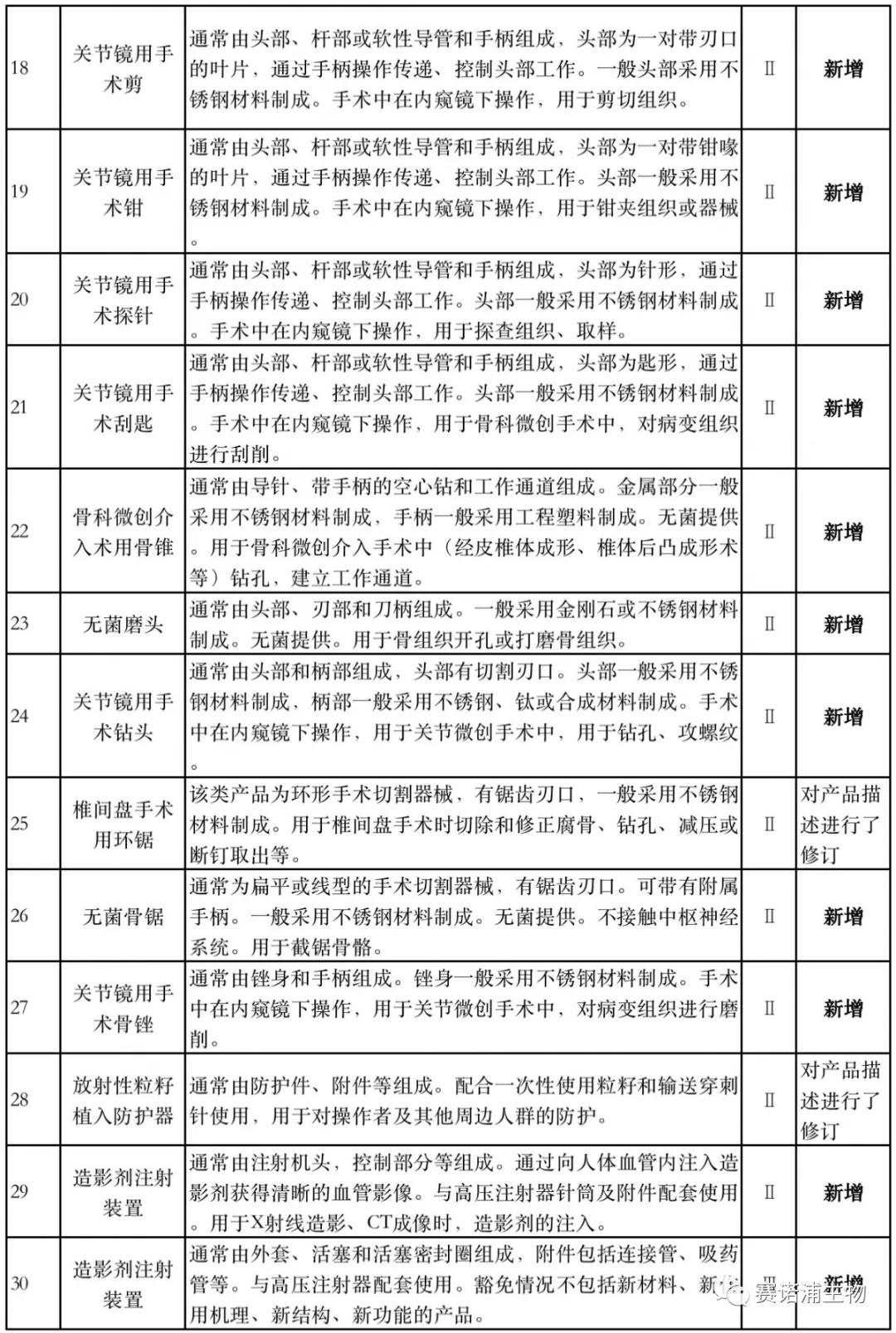
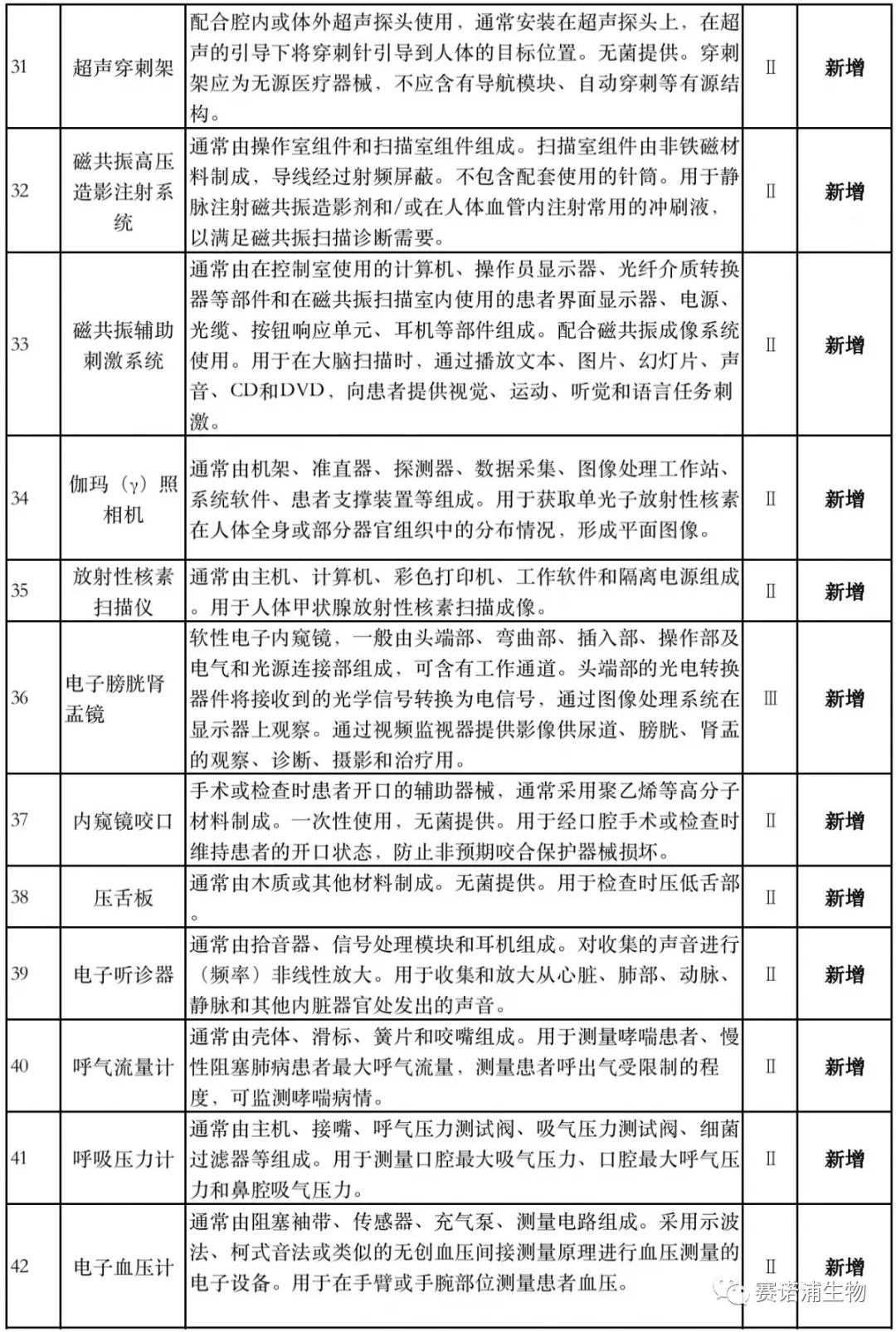
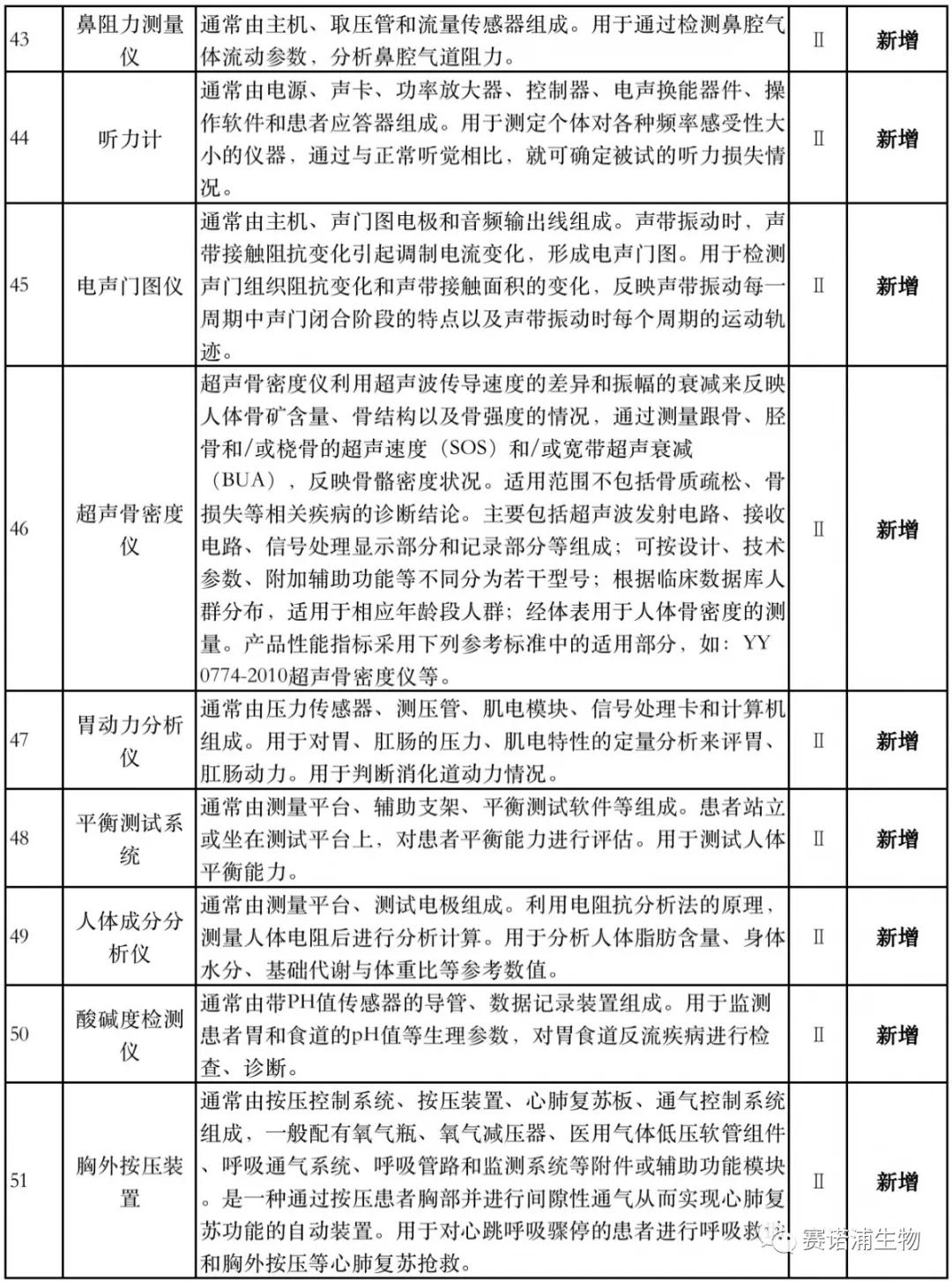
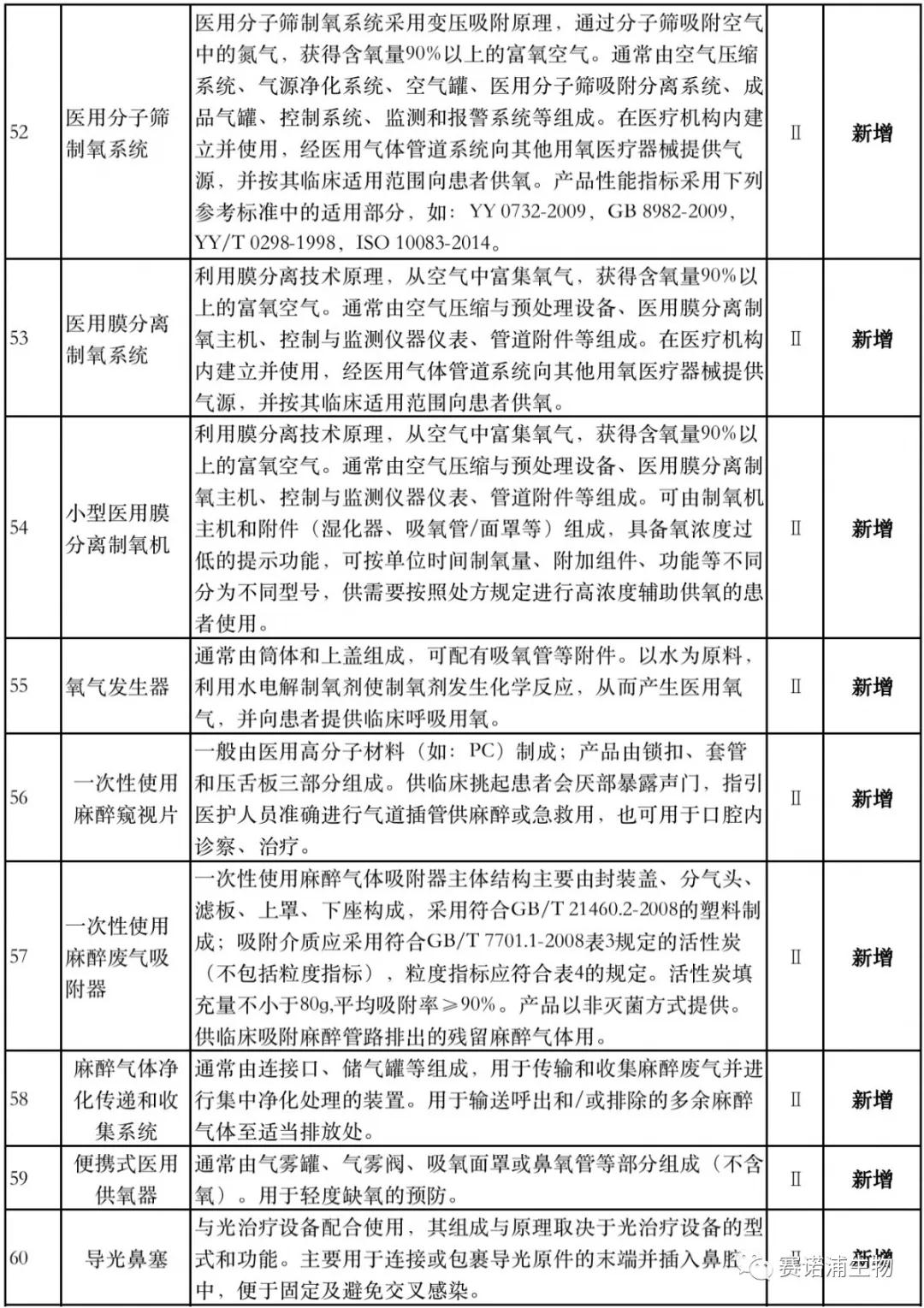
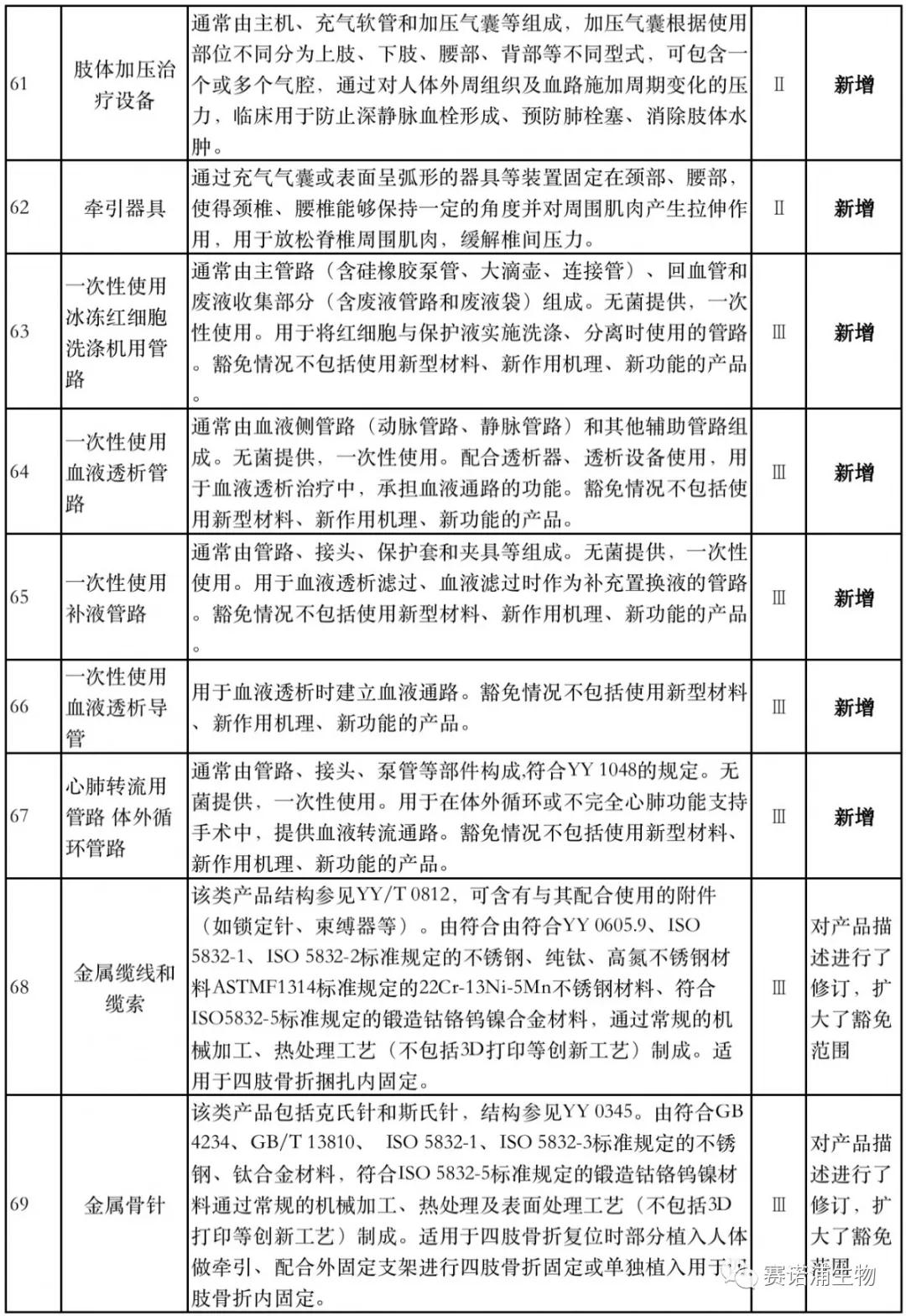
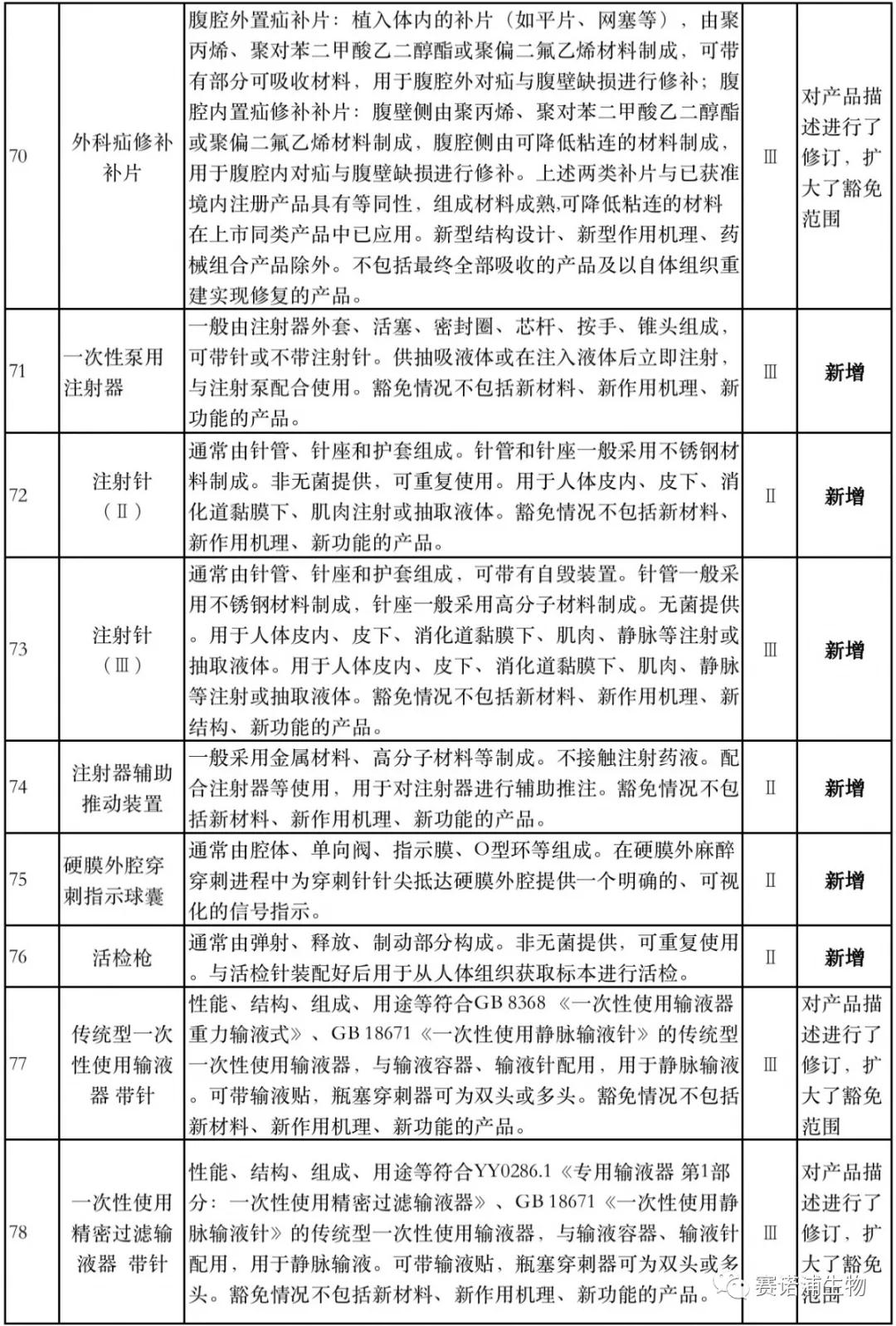
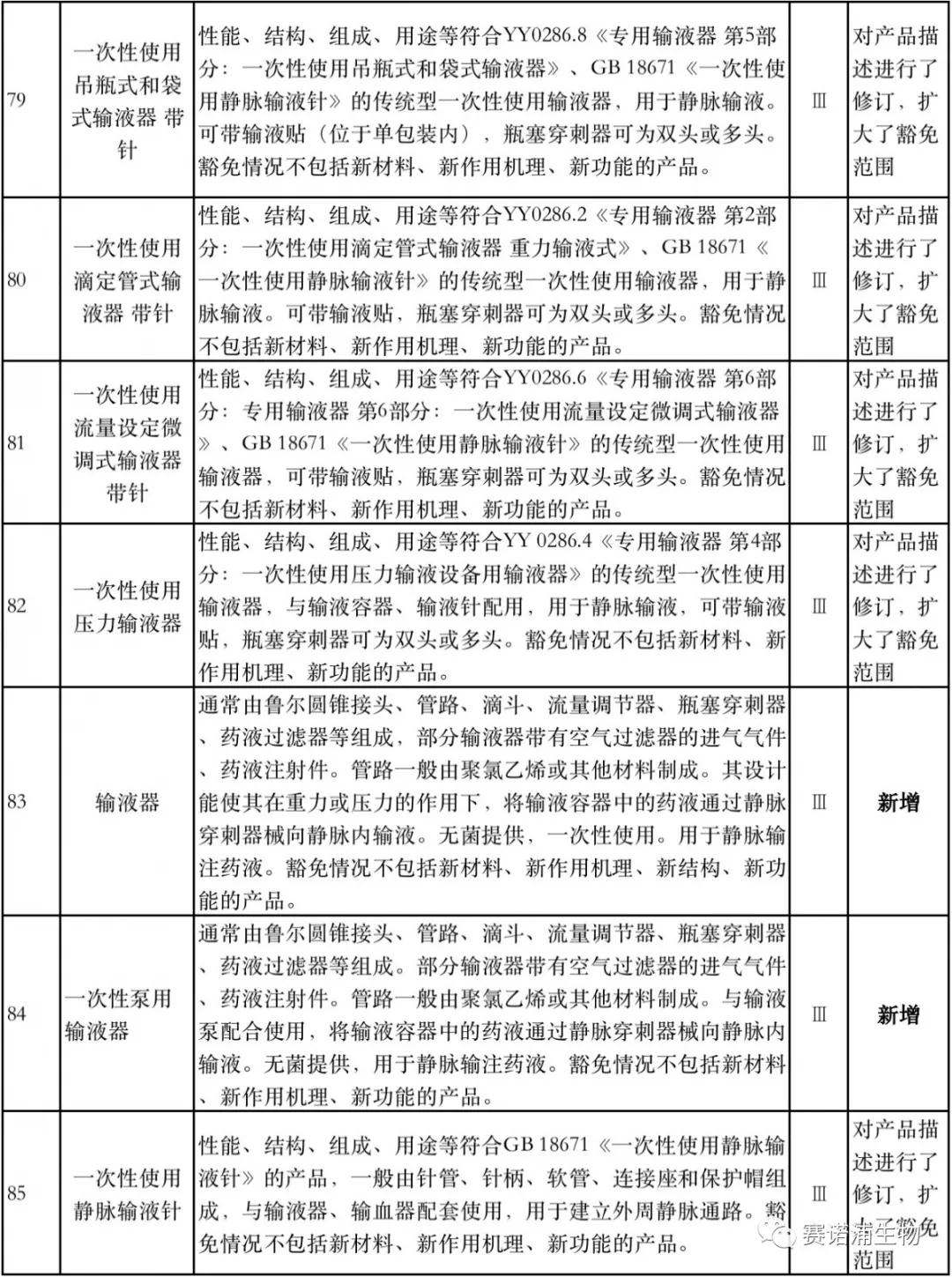
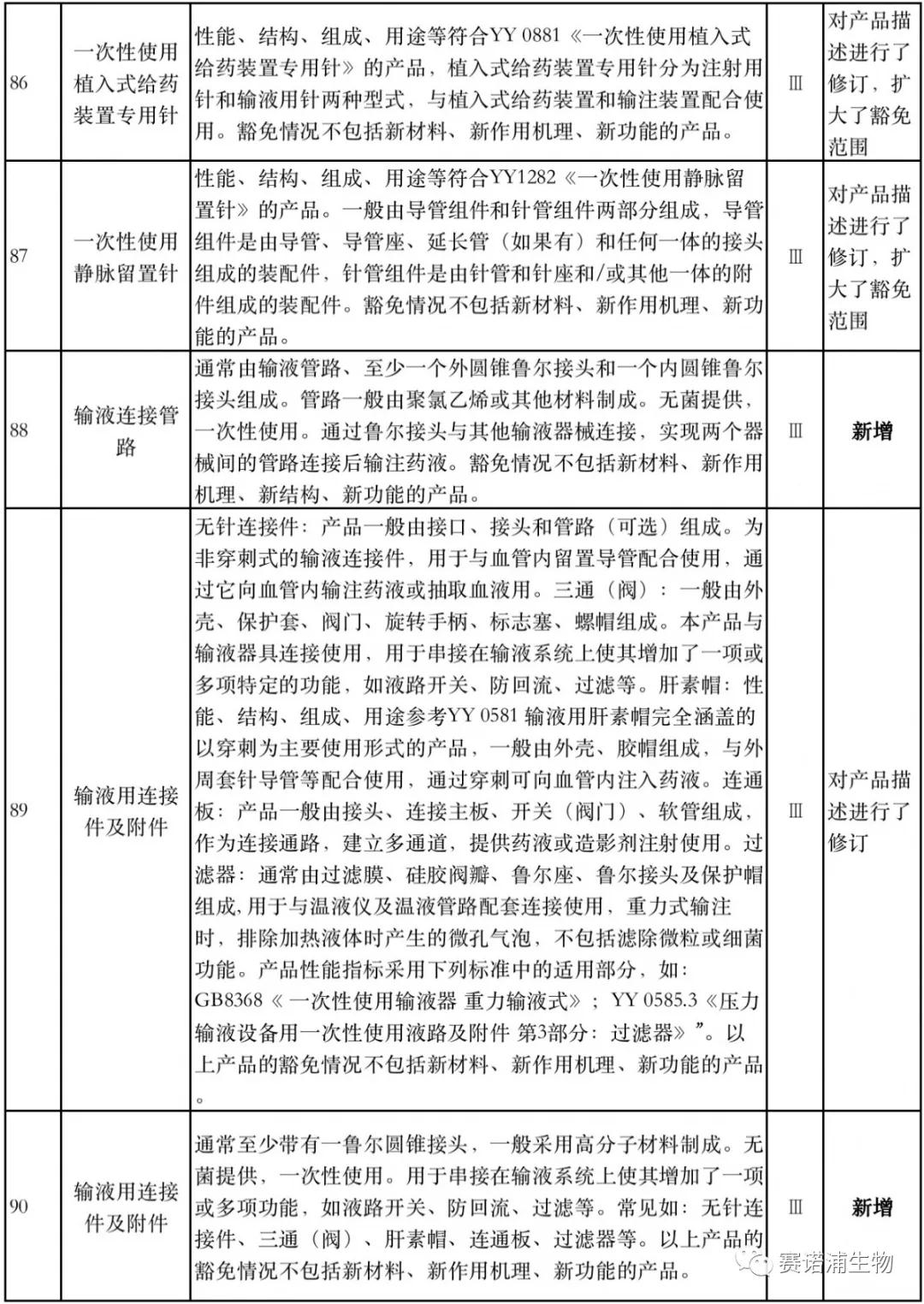
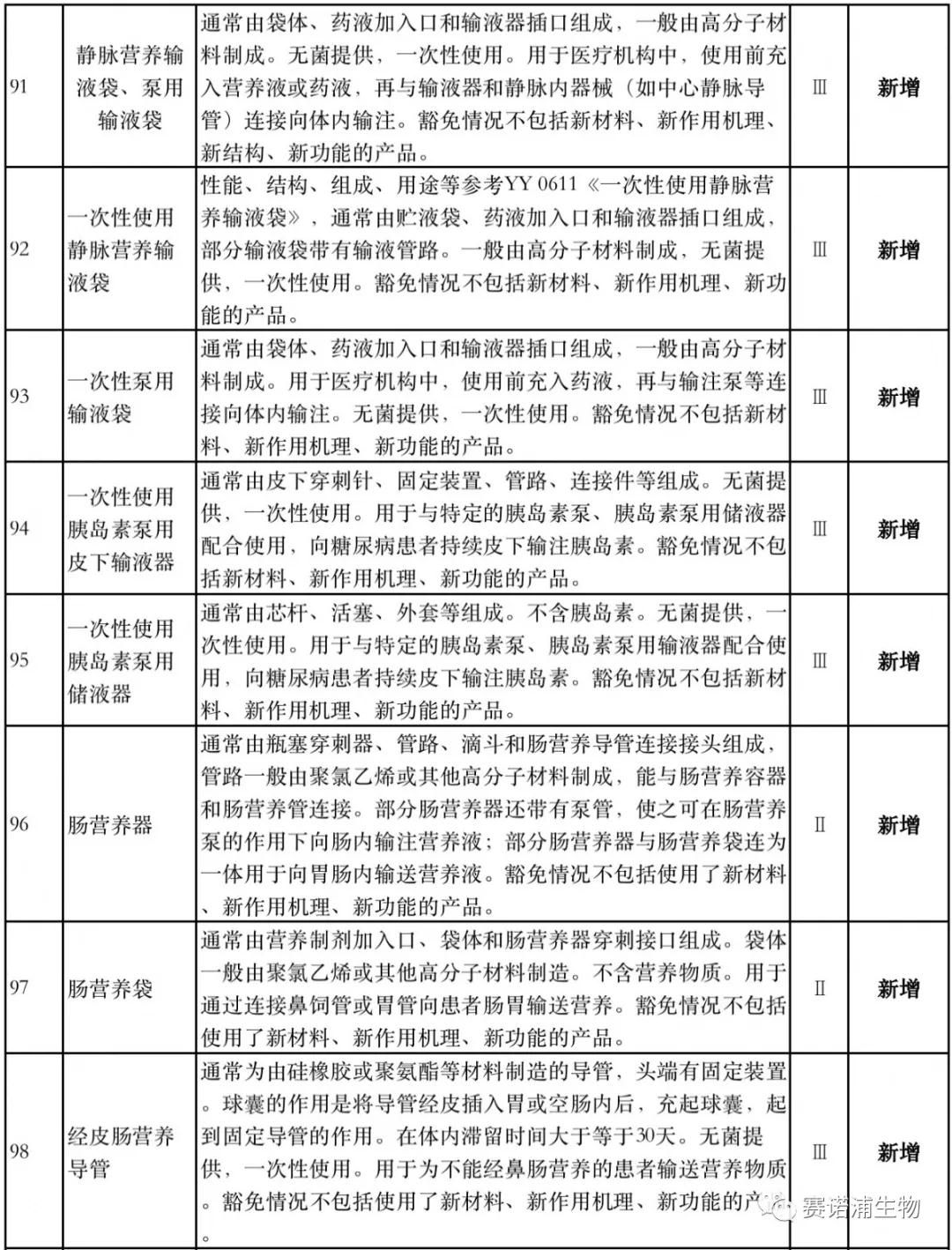
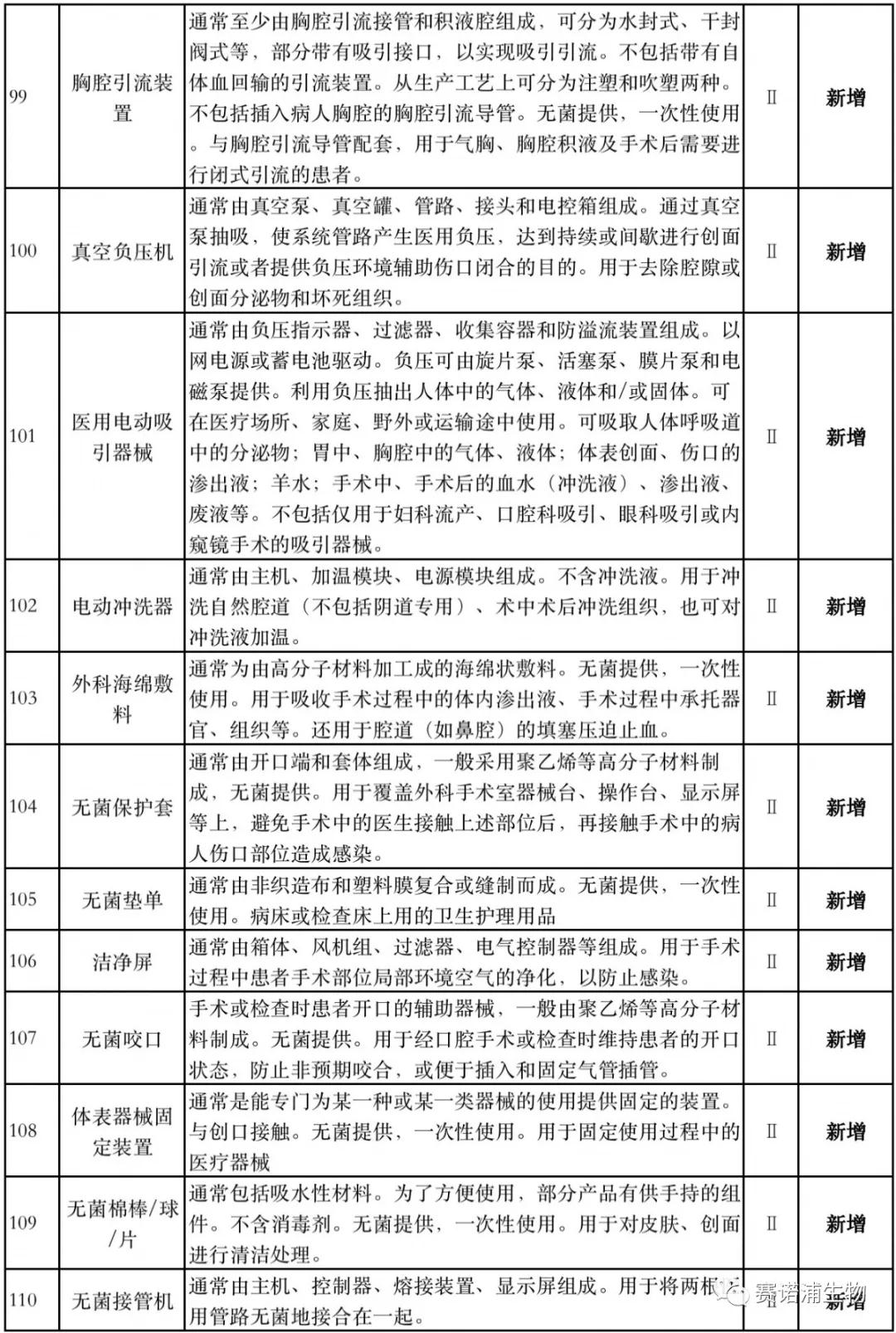
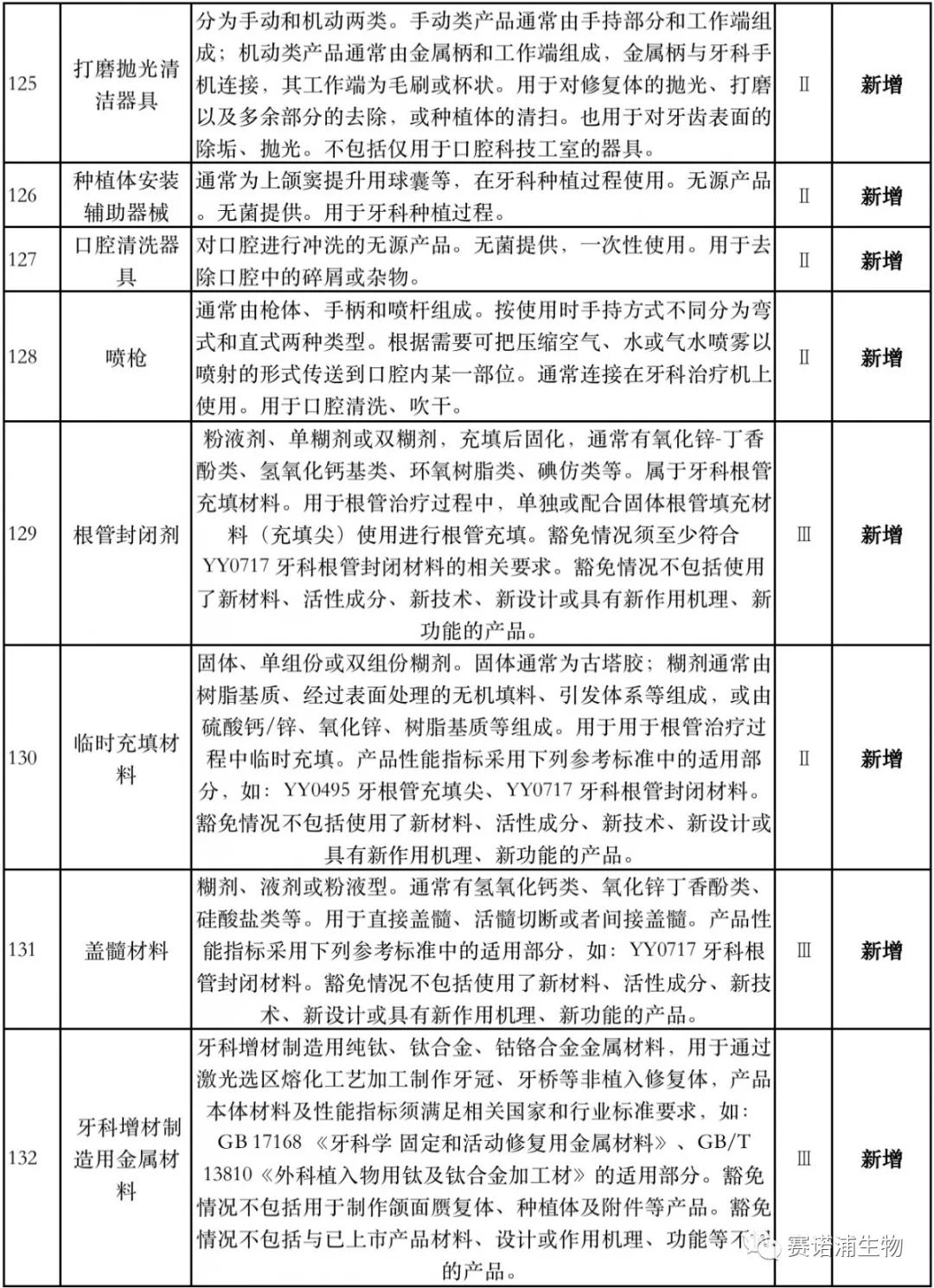
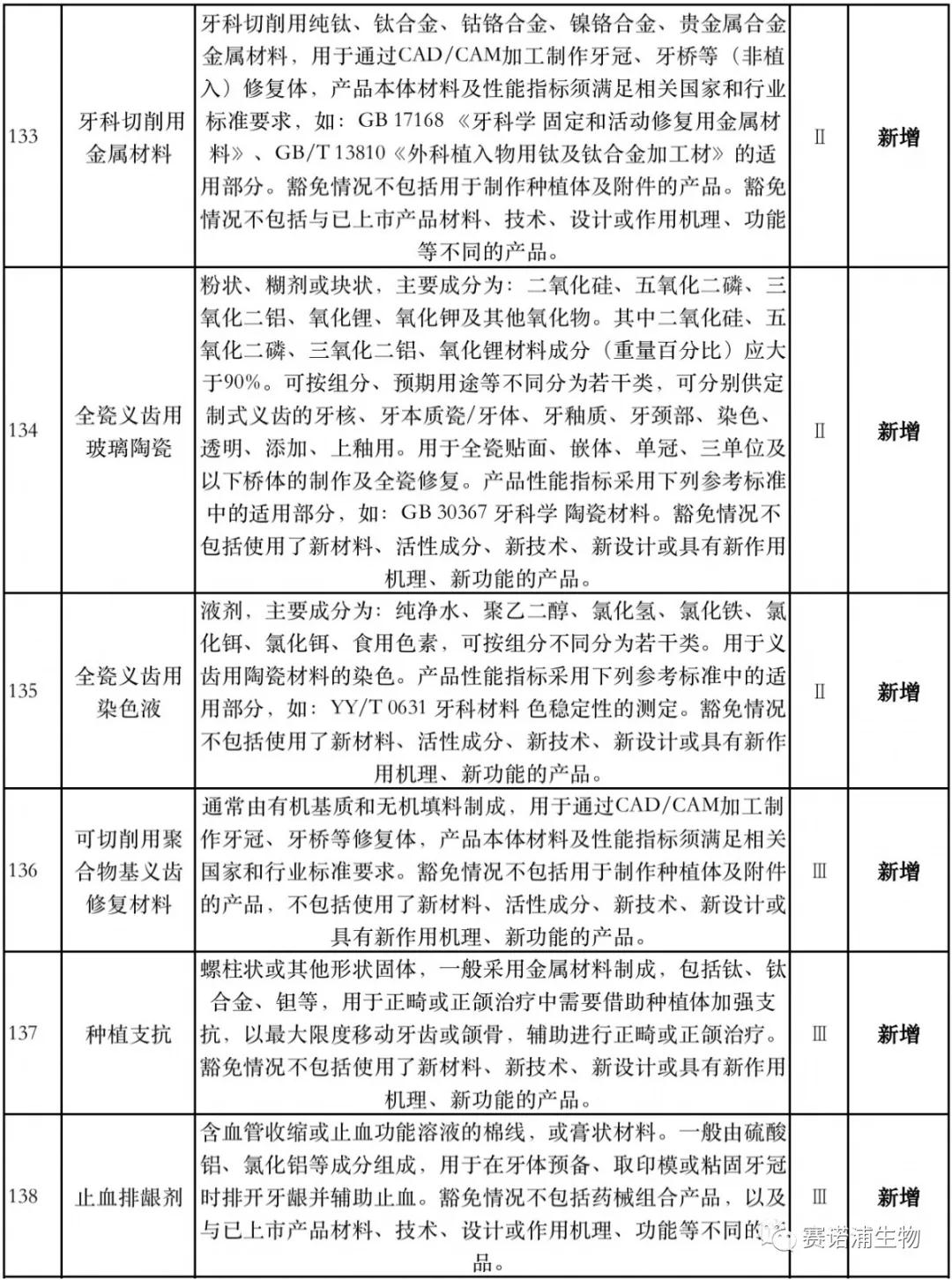
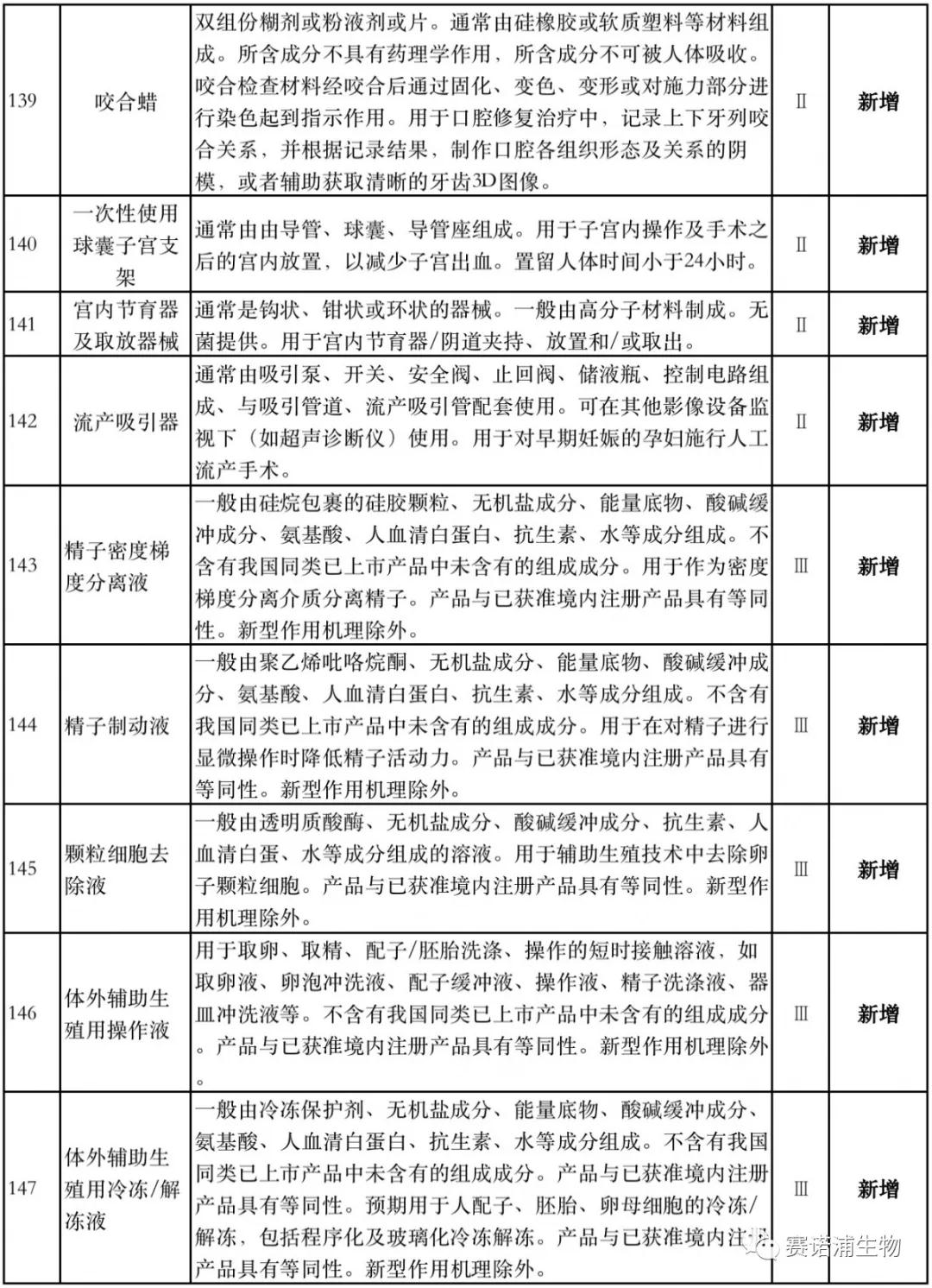
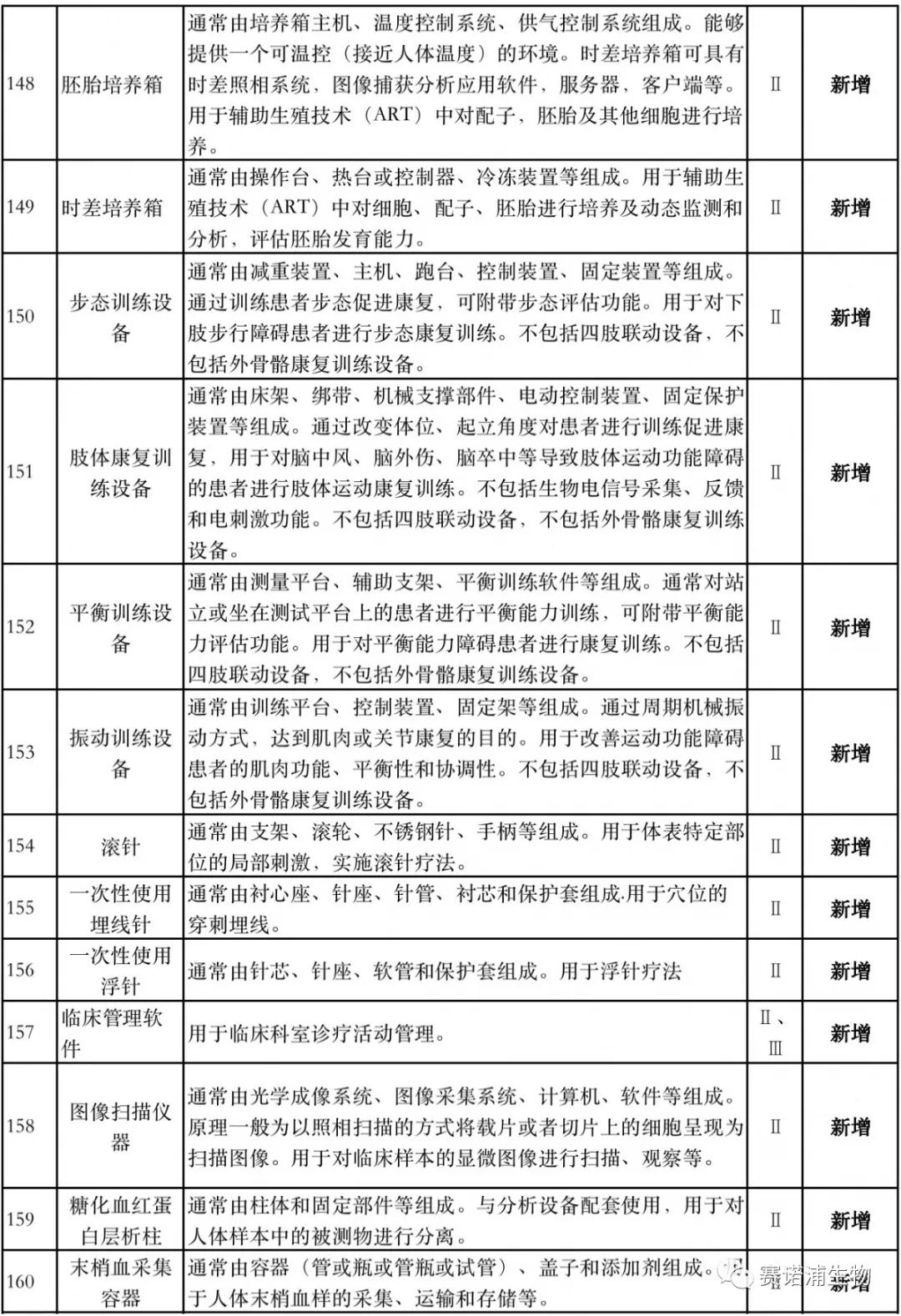
According to Xiaobian statistics, compared with the 2018 version, the 2019 version of the Exemption Catalogue (Opinion Draft) added 142 medical devices and 27 in vitro diagnostic reagents, and expanded the exemption scope or revised the description for 19 medical devices. The new 142 medical devices include 102 Class II and 40 Class III medical devices.
In fact, this is the fifth batch of medical devices that are exempt from clinical trials. The 2018 version of the Exemption Catalog contains the first four batches, a total of 1,248, including 855 medical devices and 393 in vitro diagnostic reagents, an increase from the first three batches. 84 medical devices and 277 in vitro diagnostic reagents.
Continuously expand the catalogue and increase the medical equipment exempted from clinical trials, which can effectively reduce the clinical trial requirements of products with high maturity and low risk, and reduce the burden on enterprises, so that enterprises can put more energy into product development and quality. Upgrade.
At the same time, it is also conducive to optimizing the resources for clinical trials and review and approval, investing valuable resources in clinical urgent needs and innovative medical device products, and promoting the safe, effective and risk-controlled products to be listed as soon as possible.
Attachment: New medical devices and in vitro diagnostic reagents exempt from clinical trials